


Virtual Orbitals 3D Chemistry

Perihal Virtual Orbitals 3D Chemistry
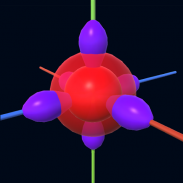
It is impossible to learn about the shapes of orbitals in a page which is 2D but the orbitals aren't 2D. Virtual Orbitals app helps you to visualize the shapes of the orbitals in 3D such that you can understand more and you can sort out your confusions.
This education app help the students to learn chemistry in a smarter way. Students can see the every parts of orbitals by rotating their fingers to screen.
Tag: Learning App, Make Chemistry Easy, Smart Education, Class 11, Class 12, Class XI, Class XII, 3D Chemistry, 3D Orbitals, Orbit, Virtual Study, App for Smart Classes, Orbital, Shape of orbitals, Atom, orbitals of all atoms, Basic of Chemistry, SPDF, S Orbital, P Orbital, D Orbital, F Orbital, How orbitals looks, atomic orbitals, Shapes of atomic orbitals, theory of orbitals, structure of atom, sub-shell, sub shells, Filling of orbitals in atom, electronic density, arrangement of electrons in atoms, arrangements of orbitals, where are electrons, electron, electronic configuration of atoms, see orbitals, observe orbitals, what are the shapes of orbitals, understanding orbitals, chemistry lovers, micro world, microscopic world, microscopic education, futuristic education, where electrons exists, molecular orbital theory, quantum physics, quantum numbers, molecular orbital diagrams, orbital diagrams, chemistry app, VSEPR Theory, Molecular Orbital Theory, h bond, h-bond, basic chemistry, organic chemistry, chemical bonding, Periodic Table, PCM, PCB, PCBM, 3D Molecules, quantum mechanism, shapes of molecules, chemistry education app, s-orbital, p-orbital, d- orbital, f-orbital, s orbital, p orbital, d orbital, f orbital.
Atoms included: Hydrogen, Helium, Lithium, Boron, Carbon, Oxygen, Neon, Sodium, Silicon, Potassium, Argon, Calcium, Zinc, Iron etc.
Ia adalah mustahil untuk belajar tentang bentuk orbital dalam halaman yang 2D tetapi orbital tidak 2D. Orbitals Maya apl membantu anda untuk menggambarkan bentuk-bentuk orbital dalam 3D seperti yang anda boleh memahami lebih lanjut dan anda boleh menyelesaikan kekeliruan anda.
Ini aplikasi pendidikan membantu pelajar untuk belajar kimia dengan cara yang lebih bijak. Pelajar boleh melihat bahagian-bahagian setiap orbital dengan memutar jari mereka ke skrin.
Tag: Pembelajaran App, Make Kimia mudah, Pendidikan Smart, Kelas 11, Kelas 12, Kelas XI, Kelas XII, Kimia 3D, 3D Orbitals, Orbit, Kajian Maya, App untuk Kelas Smart, Orbital, bentuk orbital, Atom, orbital daripada semua atom, Basic Kimia, SPDF, S orbit, P Orbital, D Orbital, F Orbital, Bagaimana orbital kelihatan, orbital atom, Bentuk orbital atom, teori orbital, struktur atom, sub-shell, cengkerang sub, Pengisian orbital dalam atom, ketumpatan elektronik, susunan elektron dalam atom, pengaturan orbital, di mana elektron, elektron, konfigurasi elektronik atom, lihat orbital, memerhati orbital, apakah bentuk orbital, memahami orbital, pencinta kimia, dunia mikro, dunia mikroskopik, pendidikan mikroskopik, pendidikan futuristik, di mana elektron wujud, teori orbital molekul, fizik kuantum, nombor kuantum, gambar rajah orbital molekul, gambar rajah orbit, aplikasi kimia, teori VSEPR, teori orbital molekul, h bon, h-bon, kimia asas, kimia organik, ikatan kimia, Jadual Berkala, PCM, PCB, PCBM, 3D molekul, mekanisme kuantum, bentuk molekul, aplikasi pendidikan kimia, s-orbit, p-orbit, d- orbit, f-orbit, s orbit, p orbit , d orbit, f orbit.
Atom termasuk: Hidrogen, Helium, Lithium, Boron, Carbon, oksigen, Neon, Sodium, Silikon, Potassium, Argon, Kalsium, zink, besi dan lain-lain


























